Risk assessment of Soulatrolide and Mammea (A/BA+A/BB) coumarins from Calophyllum brasiliense by a toxicogenomic and toxicological approach
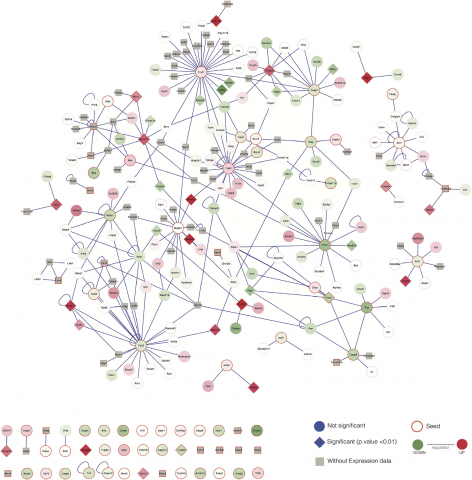
Gomez-Verjan JC, Estrella-Parra E, Vazquez-Martinez ER, Gonzalez-Sanchez I, Guerrero-Magos G, Mendoza-Villanueva D, Isus L, Alfaro A, Cerbón-Cervantes M, Aloy P, Reyes-Chilpa R, Calophyllum brasiliense (Calophyllaceae) is a tropical rain forest tree distributed in Central and South America. It is an important source of tetracyclic dipyrano coumarins (Soulatrolide) and Mammea type coumarins. Soulatrolide is a potent inhibitor of HIV-1 reverse transcriptase and displays activity against Mycobacterium tuberculosis. Meanwhile, Mammea A/BA and A/BB, pure or as a mixture, are highly active against several human leukemia cell lines, Trypanosoma cruzi and Leishmania amazonensis. Nevertheless, there are few studies evaluating their safety profile. In the present work we performed toxicogenomic and toxicological analysis for both type of compounds. Soulatrolide, and the Mammea A/BA + A/BB mixture (2.1) were slightly toxic accordingly to Lorke assay classification (DL50 > 3000 mg/kg). After a short-term administration (100 mg/kg/daily, orally, 1 week) liver toxicogenomic analysis revealed 46 up and 72 downregulated genes for Mammea coumarins, and 665 up and 1077 downregulated genes for Soulatrolide. Gene enrichment analysis identified transcripts involved in drug metabolism for both compounds. In addition, network analysis through protein-protein interactions, tissue evaluation by TUNEL assay, and histological examination revealed no tissue damage on liver, kidney and spleen after treatments. Our results indicate that both type of coumarins displayed a safety profile, supporting their use in further preclinical studies to determine its therapeutic potential.
Food and Chemical Toxicology,
2016, 91, 117-129
Pubmed: 26995226
Direct link: 10.1016/j.fct.2016.03.010
