Truncating variant burden in high-functioning autism and pleiotropic effects of LRP1 across psychiatric phenotypes
Torrico B, Shaw AD, Mosca R, Vivó-Luque N, Hervás A, Fernàndez-Castillo N, Aloy P, Bayés M, Fullerton JM, Cormand B, Toma C, Background: Previous research has implicated de novo and inherited truncating mutations in autism-spectrum disorder. We aim to investigate whether the load of inherited truncating mutations contributes similarly to high-functioning autism, and to characterize genes that harbour de novo variants in high-functioning autism.
Journal of Psychiatry & Neuroscience,
2019, 44(5), 350-359
Methods: We performed whole-exome sequencing in 20 high-functioning autism families (average IQ = 100).
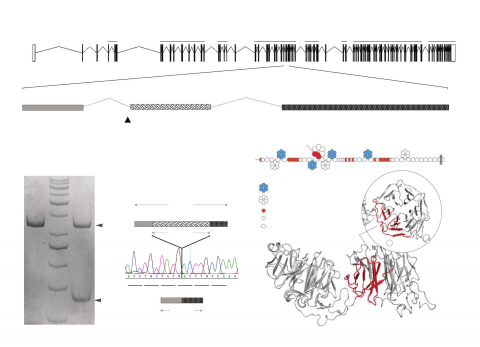
Results: We observed no difference in the number of transmitted versus nontransmitted truncating alleles for high-functioning autism (117 v. 130, p = 0.78). Transmitted truncating and de novo variants in high-functioning autism were not enriched in gene ontology (GO) or Kyoto Encyclopedia of Genes and Genomes (KEGG) categories, or in autism-related gene sets. How-ever, in a patient with high-functioning autism we identified a de novo variant in a canonical splice site of LRP1, a postsynaptic density gene that is a target for fragile X mental retardation protein (FRMP). This de novo variant leads to in-frame skipping of exon 29, remov-ing 2 of 6 blades of the β-propeller domain 4 of LRP1, with putative functional consequences. Large data sets implicate LRP1 across a number of psychiatric disorders: de novo variants are associated with autism-spectrum disorder (p = 0.039) and schizophrenia (p = 0.008) from combined sequencing projects; common variants using genome-wide association study data sets from the Psychiatric Gen-omics Consortium show gene-based association in schizophrenia (p = 6.6 × E−07) and in a meta-analysis across 7 psychiatric disorders (p = 2.3 × E−03); and the burden of ultra-rare pathogenic variants has been shown to be higher in autism-spectrum disorder (p = 1.2 ×E−05), using whole-exome sequencing from 6135 patients with schizophrenia, 1778 patients with autism-spectrum disorder and 7875 con-trols.
Limitations: We had a limited sample of patients with high-functioning autism, related to difficulty in recruiting probands with high cognitive performance and no family history of psychiatric disorders.
Conclusion: Previous studies and ours suggest an effect of trun-cating mutations restricted to severe autism-spectrum disorder phenotypes that are associated with intellectual disability. We provide evi-dence for pleiotropic effects of common and rare variants in the LRP1 gene across psychiatric phenotypes.
Pubmed: 31094488
Direct link: https://doi.org/10.1503/jpn.180184
