A Systematic Study of the Energetics Involved in Structural Changes upon Association and Connectivity in Protein Interaction Networks
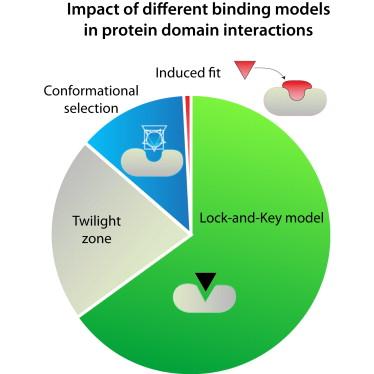
Stein A, Rueda M, Panjkovich A, Orozco M, Aloy P, The study of protein binding mechanisms is a major topic of research in structural biology. Here, we implement a combination of metrics to systematically assess the cost of backbone conformational changes that protein domains undergo upon association. Through the analyses of 2090 unique unbound → bound transitions, from over 12,000 structures, we show that two-thirds of these proteins do not suffer significant structural changes upon binding, and could thus fit the lock-and-key model well. Among the remaining proteins, one-third explores the bound conformation in the unbound state (conformational selection model) and, while most transitions are possible from an energetic perspective, a few do require external help to break the thermodynamic barrier (induced fit model). We also analyze the relationship between conformational transitions and protein connectivity, finding that, in general, domains interacting with many partners undergo smaller changes upon association, and are less likely to freely explore larger conformational changes.
Structure,
2011, 19(6), 881-9
Pubmed: 21645858
Direct link: 10.1016/j.str.2011.03.009
Link to the supplementary material
